Strain Data Sheet
RBRC02661
Strain Information | |
|---|---|
| Image | 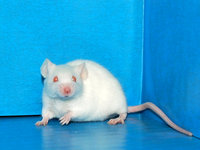 |
| BRC No. | RBRC02661 |
| Type | Congenic |
| Species | Mus musculus |
| Strain name | A.AKITA-Ins2<Akita> |
| Former Common name | Akita-Ins2 gene mutation congenic, A/J |
| H-2 Haplotype | |
| ES Cell line | |
| Background strain | A |
| Appearance | |
| Strain development | Developed by Mitsuo Itakura, The University of Tokushima and Shigeru Takeshita, Astellas Pharm Inc. in 2005. |
| Strain description | |
| Colony maintenance | |
| References | A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. Wang J, Takeuchi T, Tanaka S, Kubo S K, Kayo T, Lu D, Takata K, Koizumi A, Izumi T Journal of Clinical Investigation, 103, 27 (1999). 9884331Diabetic modifier QTLs identified in F2 intercrosses between Akita and A/J mice. Takeshita S, Moritani M, Kunika K, Inoue H, Itakura M Mamm. Genome, 17, 927-940 (2006). 16964447 |
Health Report | |
|---|---|
| Examination Date / Room / Rack | |
Gene | |||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Gene Name | Chr. | Allele Symbol | Allele Name | Common Names | Promoter | Diseases Related to This Gene |
| Ins2 MGI:96573 | insulin II | 7 | Ins2 | Akita | |||
Phenotype | |
|---|---|
| Annotation by Mammalian phenotyhpe ontology | more 73 phenotypes |
| Detailed phenotype data | |
Ordering Information | |
|---|---|
| Donor DNA | |
| Research application | Diabetes and Obesity Research covid19_progression |
| Specific Term and Conditions | In publishing the research results obtained by use of the BIOLOGICAL RESOURCE, a citation of the following literature(s) designated by the DEPOSITOR is requested. Takeshita et al., Mammalian Genome, 17, 927-940 (2006). Takeshita et al., Mammalian Genome, under submissionThe RECIPIENT of BIOLOGICAL resource shall obtain a prior written consent on use of it from the DEPOSITOR Dr. Mitsuo Itakura, Institute for Genome Research, The University of Tokushima. The RECIPIENT must send a copy of the APPROVAL FORM approved by Dr. Mitsuo Itakura to Phamacology Research Labs, Astellas Pharma Inc. (Mr. Shigeru Takeshita) by fax (+81-29-847-1536) immediately, and then send it by mail. The RECIPIENT shall obtain a license from the DEPOSITOR in the case of application for any patents or commercial use with the reslts from the use of the BIOLOGICAL RESOURCE. |
| Depositor | Astellas Pharma Inc. and Tokushima University (Astellas Pharma Inc., Tokushima University) |
| Strain Status |  Frozen embryos Frozen embryos Frozen sperm Frozen sperm |
| Strain Availability | Recovered litters from cryopreserved embryos (2 to 4 months) Cryopreserved sperm (within 1 month) Cryopreserved embryos (within 1 month) |
| Additional Info. | Necessary documents for ordering:
Genotyping protocol -PCR- |
BRC mice in Publications |
|---|
| No Data |